The 20th anniversary edition of the EHFG took place 4-6 October 2017 in Bad Hofgastein, Austria, gathering over 500 participants.
The EHFG is the most important health-related event in the European Union and a meeting point for experts from different fields within the health system. Different knowledge and different experiences are brought together to the table for discussion, differing greatly from other events which are generally focused on very specific areas.
 Prof. Maurizio Scarpa was invited to actively participate to the meeting organised by DG Research and Innovation (DG RTD), European Commission, to represent the MetabERN and particularly contributing to the Gastein session “Personalising healthcare: How rare diseases pave the way” that took place on Wednesday 4 October 2017 from 09.00 to 11.00 am.
Prof. Maurizio Scarpa was invited to actively participate to the meeting organised by DG Research and Innovation (DG RTD), European Commission, to represent the MetabERN and particularly contributing to the Gastein session “Personalising healthcare: How rare diseases pave the way” that took place on Wednesday 4 October 2017 from 09.00 to 11.00 am.
Other invited panellists that took part to the discussion were
- OLAF RIESS NeurOmics Project Coordinator, University of Tübingen
- AIN AAVIKSOO Secretary General for E-services & Innovation, Ministry of Social Affairs, Estonia & Vice-Chair, ICPerMed
- PÁLL JÓNSSON Associate Director for Research and Development, National Institute for Health and Care Excellence (NICE)
- VINCIANE PIRARD Senior Director Public Affairs (Europe & International), SANOFI Genzyme
The session was Moderated by IRENE NORSTEDT, Head of Innovative and Personalised Medicine Unit, DG RTD, European Commission
The meeting aimed at discussing current efforts in personalised medicine that aim at bringing scientific insights into the clinic to effectively
- identify disease and predisposition for disease,
- prescribe the right therapy and determine the right dose for the right patient,
- and to better deliver timely and targeted prevention.
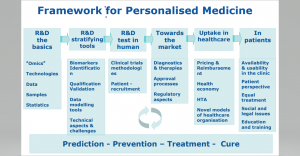 During the session the different stakeholders discuss about current and future way to exchange knowledge and develop strategies, policies and guidance that pave the way to personalised medicine in Europe, using rare diseases as a model. Advances in genomics and other “omics” technologies have significantly improved our understanding of the pathogenesis of rare diseases. This has opened avenues for piloting new, personalised diagnostic methods and therapies. The impact of “omics” is reinforced by the combination of these data with Real-World Data (RWD).
During the session the different stakeholders discuss about current and future way to exchange knowledge and develop strategies, policies and guidance that pave the way to personalised medicine in Europe, using rare diseases as a model. Advances in genomics and other “omics” technologies have significantly improved our understanding of the pathogenesis of rare diseases. This has opened avenues for piloting new, personalised diagnostic methods and therapies. The impact of “omics” is reinforced by the combination of these data with Real-World Data (RWD).
What are Omics technology? “Omics” are technologies used to characterise and quantify pools of biological molecules and to explore their roles, relationships and actions in the cells of a living creature. They are primarily aimed at the universal detection of genes (genomics), mRNA (transcriptomics), proteins (proteomics) and metabolites (metabolomics) in a specific biological sample.
What are RWD? RWD are commonly defined as information reported and collected from routine clinical settings. Notably, it has been recognised that large data sets of detailed phenotypes integrated with genetic data help adjust dosage and select therapy. RWD is also vital for post-authorisation evidence generation. The blurred boundary between clinical care and research in rare diseases makes them an excellent candidate for piloting integrated bench-to-bedside pipelines to ensure the rapid translation of research findings into clinical support for personalised medicine.
What do we mean by personalized Medicine? According to the definition developed by the Advisory group for the H2020 Health, demographic change and well-being challenge “Personalised medicine refers to a medical model using characterisation of individuals’ phenotypes and genotypes (e.g. molecular profiling, medical imaging, lifestyle data) for tailoring the right therapeutic strategy for the right person at the right time, and/or to determine the predisposition to disease and/or to deliver timely and targeted prevention”
In the field of Rare diseases the most important thing is the sharing of knowledge and experience in order to do the best for the patients. European Reference Networks(ERNs) are therefore very important.
What are ERNs? European Reference Networks are virtual networks involving healthcare providers across Europe. They aim to facilitate discussion on complex or rare diseases and conditions that require highly specialised treatment and a concentration of knowledge and resources. There are 24 ERNS involving 25 European countries plus Norway, over 300 hospitals with over 900 healthcare units and covering all major disease groups. ERNs are patients centered. In the specific case, MetabERN and its members, for example, demonstrate and document their patient-centred approach and commitment to patient empowerment having them deeply involved in the organizational structure (governance) of the network and taking particularly into account any input and experience of patients, families and patients’ association to be used in the development of guidelines and pathways, quality and safety framework, outcome and performance indicators. Moreover MetabERN is particularly focused at creating awareness and new generation of doctors that can help the patients to get a timely diagnosis.
How can the ERNs improve the lives of patients? Between 6 000 and 8 000 rare diseases affect an estimated 30 million people in the EU. An unfortunate feature of rare diseases and complex conditions is the scarcity and fragmentation of specialist knowledge, which is often not available in the patient’s region or country. Many patients therefore do not find a satisfactory explanation for their symptoms or the necessary knowledge on treatment options. By consolidating knowledge and expertise scattered across countries, the ERNs will give healthcare providers access to a much larger pool of expertise. This will result in better chances for patients to receive an accurate diagnosis and advice on the best treatment for their specific condition.
How to make the data usable? It is important to have digitalized data and patients’ information: we need to extend access to data at regional, national or across the borders level. To facilitate and create research it is needed politic will, interoperability, technical standards, semantic, legal agreement, data protection and organizational interoperability. This are major issues for the ERNs since data sharing is fundamental in the context of ERNs. To this aim the European Commission is preparing a platform meant to help the 300 Health Care Providers to share data. This constitutes an important step forward toward the operability of data since it will be interfacing with already existing platforms. This is a very important moment since we are assisting to convergence of different disciplines coming together and of different stakeholders willing to start to work together and to the establishment of a number of initiatives (IRDIC, E-rare, digitalization of health and care, health technology and assessment, etc) that support collaborative research projects.
Video recordings of all the EHFG sessions are available on the Gastein website: https://webcasting.streamdis.eu/Mediasite/Catalog/Full/


 This meeting was aimed to create collaboration among the Italian HCPs involved in the European Reference Networks (ERN), that as highlighted by Andrzej Jan Rys (Director HelathSystems, Medical Products and Innovation, DG Sante’) are virtual networks of specialists officially born in March 2017, dedicated to the diagnosis and treatment of diseases complex or rare. The 24 ERNs so far approved cover all major disease groups and represent almost a thousand of multidisciplinary medical teams of more than 300 hospitals, located in 25 EU Member States and Norway.
This meeting was aimed to create collaboration among the Italian HCPs involved in the European Reference Networks (ERN), that as highlighted by Andrzej Jan Rys (Director HelathSystems, Medical Products and Innovation, DG Sante’) are virtual networks of specialists officially born in March 2017, dedicated to the diagnosis and treatment of diseases complex or rare. The 24 ERNs so far approved cover all major disease groups and represent almost a thousand of multidisciplinary medical teams of more than 300 hospitals, located in 25 EU Member States and Norway.

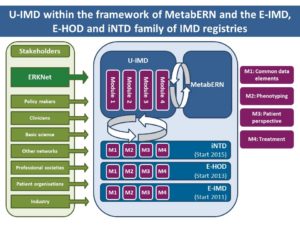 s to establish the first unified European registry that encompasses all inherited metabolic disorders (IMDs) as listed by Orphanet (http://www.orpha.net/). The overall aim of this project is to promote health for children, adolescents and adults affected by rare IMDs and to reduce variation between countries and enable and empower patients, wherever they live, to access the necessary expertise and services and promote research on IMDs and the development of safe and efficacious new treatments. Furthermore, U-IMD will systematically collect data of affected individuals with an IMD of yet unidentified molecular origin and will group them according to their clinical and biochemical phenotype. This will help to identify and systematically treat and follow these patients once the etiology of their disease has been clarified.
s to establish the first unified European registry that encompasses all inherited metabolic disorders (IMDs) as listed by Orphanet (http://www.orpha.net/). The overall aim of this project is to promote health for children, adolescents and adults affected by rare IMDs and to reduce variation between countries and enable and empower patients, wherever they live, to access the necessary expertise and services and promote research on IMDs and the development of safe and efficacious new treatments. Furthermore, U-IMD will systematically collect data of affected individuals with an IMD of yet unidentified molecular origin and will group them according to their clinical and biochemical phenotype. This will help to identify and systematically treat and follow these patients once the etiology of their disease has been clarified.



 Prof Scarpa main key tasks are the following:
Prof Scarpa main key tasks are the following: 24 European Reference Networks (ERNs) were formally launched on 9 March 2017. Representatives from the networks and Member States, along with patients and policymakers, gathered in Vilnius, Lithuania, for the 3rd ERN Conference and kick-off meetings (9 & 10 March).
24 European Reference Networks (ERNs) were formally launched on 9 March 2017. Representatives from the networks and Member States, along with patients and policymakers, gathered in Vilnius, Lithuania, for the 3rd ERN Conference and kick-off meetings (9 & 10 March).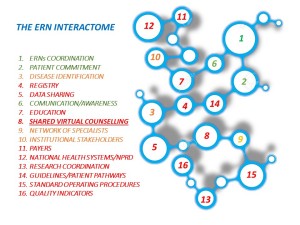 ERNs. “There is crosslinking in nature,” said Dr Scarpa. “Nothing is isolated.” He said ERNs should be at the frontier of ‘network medicine’ – a new way of thinking of health which breaks down silos between disciplines and disease taxonomies. Tools must be developed to link the ERNs, including a shared virtual counselling system that allowed members of different ERNs to discuss complex cases. “ERNs are not a project or a programme they are a concept – a revolution in the understanding of rare diseases,” he said. “Don’t think of ERNs as discrete networks but as a large task force for sharing expertise.”
ERNs. “There is crosslinking in nature,” said Dr Scarpa. “Nothing is isolated.” He said ERNs should be at the frontier of ‘network medicine’ – a new way of thinking of health which breaks down silos between disciplines and disease taxonomies. Tools must be developed to link the ERNs, including a shared virtual counselling system that allowed members of different ERNs to discuss complex cases. “ERNs are not a project or a programme they are a concept – a revolution in the understanding of rare diseases,” he said. “Don’t think of ERNs as discrete networks but as a large task force for sharing expertise.”

